|
|
|
Towa Pharmaceutical Co., Ltd.
|

|
Vision/Mission
We contribute to people’s health.
We are dedicated to people’s genuine smile.
The Towa group contributes to people’s health through the creation of excellent products and service.
We aim to become a corporation heartily appreciated and desired by patients, medical professionals, local community, and other people through our corporate activities.
Company Goals & Objectives
Towa will supply high quality generic drugs that people can trust in their medical requirements and will meet expectations and gain trust of patients and medical professionals.
The Japanese government has established a goal to increase the use of generic drugs to more than 80% until the end of 2020 and we expect that it will grow our sales. We have to establish and consolidate a framework in order to handle the increase in demand of generic drugs.
In addition, it is said that generic drugs could face their peak around 2045 in accordance with the rate of the elderly in the population, and its demand would gradually decrease after its peak. As a way of overcoming the damage of the decrease in demand, Towa is considering focusing more on overseas business. We aim to expand the Towa brand to overseas and wish that our value-added products will make people smile all over the world.
Business Description
Towa, being a pharmaceutical company specialized in generic drugs, has been dedicated to research and development, production and marketing of generic drugs for more than 65 years. Generic drugs have increasingly played a very important role in this ageing society because they are economically affordable for patients and help to curtail the financial burden of the government.
In addition to promoting stable supply, quality assurance, and information about generic drugs, Towa focuses on research and development of value-added formulations that are easily taken by patients and handled by medical professionals.
Furthermore, Towa has established the "Towa Direct Sales System," i.e. the sales network of sales offices and agents throughout Japan, which ensures customers’ confidence in using generic drugs manufactured by Towa.
Towa will achieve sound growth with widespread use of generic drugs in society and believes that it will meet people’s expectations.
Competitive Advantages
Towa has mainly three competitive advantages: value-added technology, high quality and stable supply.
We developed our original orally disintegrating tablets (OD tablets) technology called "RACTAB" which is named after "Rapid And Comfortable Tablets". OD tablets, which can rapidly and easily disintegrate in the mouth without the need for water, are widely developed by many pharmaceutical companies. What differentiates RACTAB from ordinary OD tablets is that it has an excellent bitterness masking and sufficient hardness. We have received awards for this unique technology.
Towa manufactures the products under stringent controls and global standards. We also control our product quality from active pharmaceutical ingredients (API) and the quality of API is one of our focuses now.
And thanks to the large capacity at our three plants, we can supply the products without stock-out, which pharmaceutical companies should avoid the most.
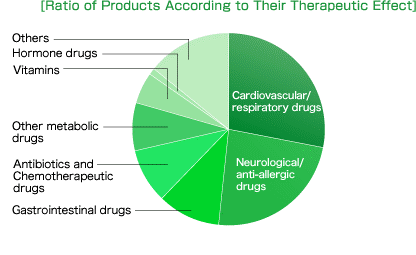
Products/Services
694 generic drugs (as of June 2016)
Markets
All around Japan.
Overseas: Korea, Taiwan, Mongolia, Philippines and Hong Kong.
Industry
Manufacture and sale of generic drugs.
Location
Headquarters:
2-11, Shinbashi-cho, Kadoma, Osaka 571-8580 Japan
Osaka Plant:
3-8 Matsuo-cho, Kadoma, Osaka 571-0044 Japan
Okayama Plant:
34-2 Taiheidai, Shoo-cho, Katsuta-gun, Okayama 709-4321 Japan
Yamagata Plant:
17-8, Aza Yuzakayama, Kanakame, Kaminoyama, Yamagata 999-3101 Japan
Financial Projection
Net sales is 82.1 billion yen as of March 31, 2016, and we estimate they will reach 93.5 billion yen in 2017.
Management
President: Itsuro Yoshida
Senior Managing Director: Takashi Osawa
Managing Director: Toshio Shirakawa
Directors: Keiji Yabushita, Yoshiaki Nishikawa, Sadayuki Morino, Shigeru Maeyama, Kazuhiko Konno, Kazuto Okimoto, Takashi Mukuta, Yasushi Naito, Satoru Nagamura
Outside Director: Norikazu Eiki
Employees
2,232 employees (as of April 1, 2016)
Highlights
1951 Towa Pharmacutical Company is established at Awaji-cho, Higashi-ku, Osaka.
1957 Upon relocation to Dosho-machi, Higashi-ku, Osaka, Towa Pharmacutical Co., Ltd (abbreviated as Towa) is established and incorporated. The Gamo Plant is constructed at Gamo-cho, Jyoto-ku, Osaka.
1964 Neyagawa Plant is constructed at Kamikamida, Neyagawa, Osaka. Gamo Plant is closed and unified to Neyagawa Plant.
1974 Towa moves its corporate headquarters to new office in Matsuo-cho, Kadoma, Osaka.
1975 The Kadoma Plant is constructed at Kuwazai-shinmachi, Kadoma, Osaka.
1978 The Osaka Plant is constructed at the headquarters site. Neyagawa Plant is unified to Osaka Plant and Kadoma Plant. Kadoma Laboratory is constructed at Kuwazai-shinmachi, Kadoma, Osaka. Distribution Center is constructed in Fukata-cho, Kadoma, Osaka.
1982 Osaka Plant Annex is constructed at Yanagida-cho, Kadoma, Osaka. Kadoma Laboratory enlarges to conform to GLP.
1983 The Okayama Plant is constructed at the Shoo Industrial Park in Okayama.
1984 Towa acquires Beppu Hot Spring Science Institute Ltd., renames it Oita Plant, and starts manufacturing injections there.
1987 Kadoma Plant is closed and unified to Osaka Plant and Okayama Plant.
1989 Three sales company, Towa Pharmaceutical Sales Co., Ltd., Hanshin Towa Pharmaceutical Sales Co., Ltd., and Towa Pharmaceutical Tokyo Sales Co., Ltd. are consolidated.
1994 Towa’s shares are listed on the over-the-counter market of the Japan Securities Dealers Association.
1995 Distribution Center is constructed at the Okayama Plant site.
1997 Towa moves its corporate headquarters to newly built office in Shinbashi-cho, Kadoma, Osaka.
1998 Osaka Research Center and Osaka Distribution Center are constructed at Ichiban-cho, Kadoma, Osaka. Towa purchases Mect Co., Ltd.’s Tohoku Plant, re-opens it as Yamagata Daiichi Plant.
2003 Towa purchases J-Dolph Co., Ltd., and makes it a subsidiary.
2004 Towa’s shares are listed on the second of Tokyo Stock Exchange (TSE).
2005 Listing of Towa’s share transfers to the first section of TSE.
2006 Osaka Daini Plant is closed and unified to the Osaka plant which is constructed at Matsuo-cho, Kadoma, Japan.
2009 Oita Plant is closed and unified to the Okayama plant.
2010 Towa purchases Daichi Kasei Co., Ltd., and makes it a subsidiary.
2012 Yamagata Plant is constructed at Kaminoyama, Yamagata. East Japan Distribution Center is constructed at Kaminoyama, Yamagata. West Japan Distribution Center is constructed at the Shoo Industrial Park in Okayama. Osaka and Okayama Distribution Center are closed.
2014 Yamagata Daiichi Plant is closed.
Notable Developments
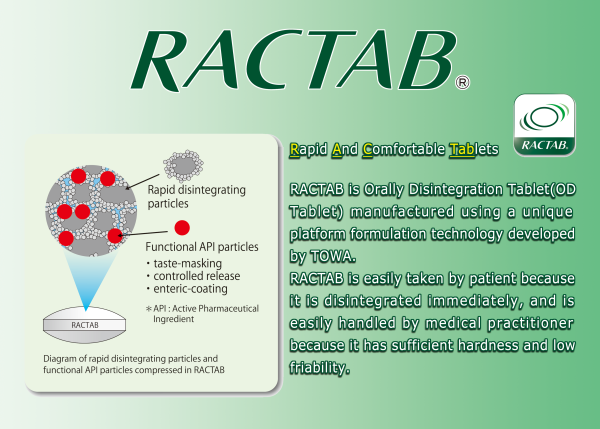
Formulation Design of RACTAB
RACTAB is a tablet prepared by dry compression of the following two kinds of particles:
• Rapid disintegrating particles – prepared by coating the surface of the saccharides with corn starch and crospovidone;
• Functional active pharmaceutical ingredient (API) particles – prepared by mixing API particles or with various functionalities with the saccharides coated with corn starch and crospovidone.
API particles with various functionalities, such as taste masking, controlled release and enteric coating, are prepared depending on the characteristics of the API.
As announced in April 2017, the forecasts for the fiscal year ending March 31, 2018 ("FY2017") (core basis) are as follows: The sales forecast is 1,279.0 billion yen (-2.5% year-on-year).
By microspheric coating of corn starch and crospovidone on the saccharide surface, the functionality of the saccharide changes, and the particles come to have a regular cavity structure like Ishigaki (stone wall) even after pressure molding.
It is speculated that the high hardness of RACTAB is attributed to very tight binding between the contact areas of particles because the particles’ surfaces are coated with corn starch and crospovidone, giving it high connectivity.
Furthermore, RACTAB is disintegrated easily and immediately in the oral cavity because of the high water absorption capacity of corn starch and strong disintegrating function of crospovidone.
Website
http://www.towayakuhin.co.jp/english/
|
|
|
|